

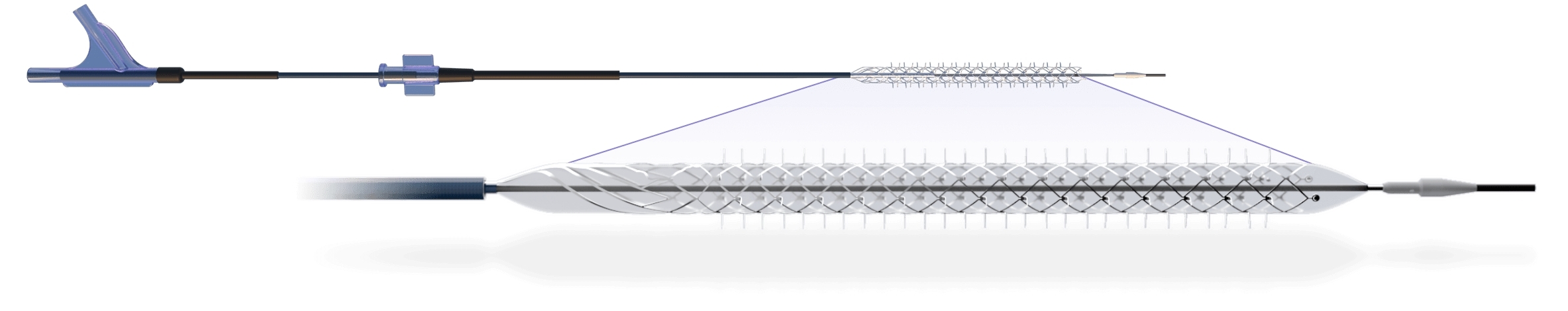
“Completing enrollment is a significant milestone in the clinical validation and regulatory pathway to approval of the Spur Stent System to treat patients,” said Teo Jimenez, Vice President of Research and Development for Reflow Medical. Intended for use in conjunction with a commercially available drug-coated balloon (DCB), the Temporary Spur Stent features a self-expanding nitinol scaffold with radially expanding spikes designed to penetrate the diseased vessel wall and enhance drug delivery, without leaving anything behind.Īccording to Michael Lichtenberg, MD, FESC, Chief Medical Officer of the Angiology Department at Vascular Centre Clinic, Arnsberg, Germany, and a Principal Investigator for the trial, “The temporary mechanical scaffolding, followed by a DCB, has the potential to minimize vessel recoil and boost luminal gain.” He continues, “The fact that it does so without leaving anything behind means the patient avoids the risk of complications from the use of a stent.”
#REFLOW MEDICAL TRIAL#
announces that it has completed patient enrollment in the DEEPER OUS clinical trial (NCT03807531) for the company’s Temporary Spur Stent System, a novel device that features a retrievable stent designed for complex infrapopliteal disease.ġ06 patients have now been enrolled in the prospective, nonrandomized trial in multiple centers in Europe and New Zealand.
SAN CLEMENTE, Calif.-( BUSINESS WIRE)-Reflow Medical, Inc.


 0 kommentar(er)
0 kommentar(er)
